Perché reagiscono le molecole e come avvengono le reazioni?
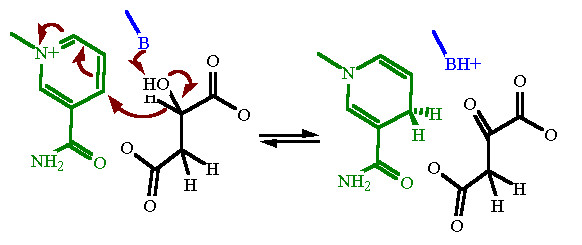
Perché reagiscono le molecole? Perché avvengono le reazioni chimiche? La chimica che è la scienza del cambiamento ha la risposta in due suoi grandi capitoli: la termodinamica e la cinetica, due concetti fondanti della disciplina. La termodinamica ci informa della stabilità energetica delle molecole. Essa tratta con il calore, il… Read More »Perché reagiscono le molecole e come avvengono le reazioni?